Abstract
Introduction: Symptom management is considered a top priority of cancer care. Monitoring patient-reported outcomes (PROs) such as side effects of treatment has been shown to improve quality of life and even prolong survival among cancer patients. For regulators, PROs collected from patients and the caregivers who speak for patients when they are too ill or unable to speak for themselves informs patient-focused drug development (PFDD). PFDD is a key-component of the 21st Century Cures Act that aligns FDA, industry, advocacy and academic partners to accelerate medical product development for those who need it most. We sought to understand the prevalence of side effects experienced by people with acute myeloid leukemia (AML) and to assess differences in the report of side effects from patients across the disease trajectory, including those who were deceased or too ill to directly self-report.
Methods: We assessed patient reported prevalence of severe short- and long-term side effects from AML treatments as a part of a national survey. Caregiver reports were included so as to capture the experiences of patients who had either passed away from AML or who were too ill to participate. Participants rated their experience with short-term side effects (nausea, infection, mouth sores, diarrhea, hair loss) and long-term side effects (organ dysfunction, neuropathy, fatigue, chemo brain) as 'none,' 'mild,' 'moderate,' or 'severe.' Prevalence of severe side effects was assessed using descriptive statistics, and differences in likelihood of reporting severe side effects were assessed using logistic regression.
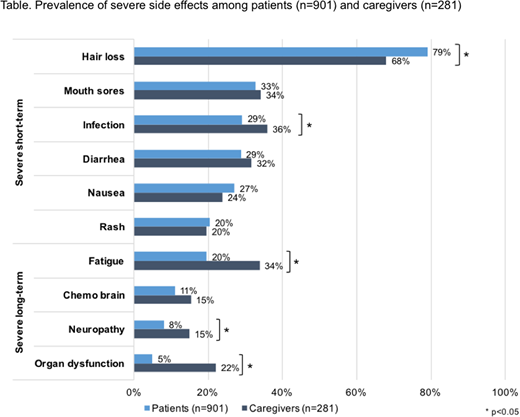
Results: A total of 1182 eligible participants completed the survey, including 901 patients and 281 caregivers. Participants were an average of 55 years old (SD=13.0), and 12% were non-white minorities. Patient participants had been diagnosed with AML for 7.7 years (SD=4.7), while patients reported on by caregiver participants had been diagnosed for 5.7 years (SD=4.1). Almost all participants received chemotherapy (98%), and one-fifth received radiation (21%). Hair loss was the most common severe short-term side effect among caregivers (68%) as well as patients (79%, p=0.01). Conversely, patients were less likely than caregivers to report a severe infection (29% vs 36%, p<0.001). Patients and caregivers reported similar rates of other short-term side effects, including mouth sores (33 % vs 34%, p=0.22), diarrhea (29% vs 32%, p=0.07), nausea (27% vs 24%, p=0.26), and rash (20% for both; p=0.80). Patients were consistently less likely to report severe, long-term side effects as compared to caregivers, including fatigue (20% vs 34%, p<0.001), neuropathy (8% vs 15%, p=0.02) and organ dysfunction (5% vs 22%, p<0.001). Patients and caregivers reported similar rates of severe 'chemo brain' (11% vs 15%, p=0.43).
Conclusions: AML patients of diverse disease stages experience side effects as well as long-lasting collateral damage from treatments. PRO measures can collect important prevalence data for these events. As compared to their healthier patient counterparts, caregivers reporting on deceased or ill patients demonstrated similar rates of severe short-term side effects, but higher rates of long-term, collateral damages from their treatment. Despite the difficulty in objective quantification, that over 10% of the sample reported severe 'chemo brain' validates the disease community's continued interest in better understanding this experience through PFDD.
Bridges:The Leukemia & Lymphoma Society: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.